







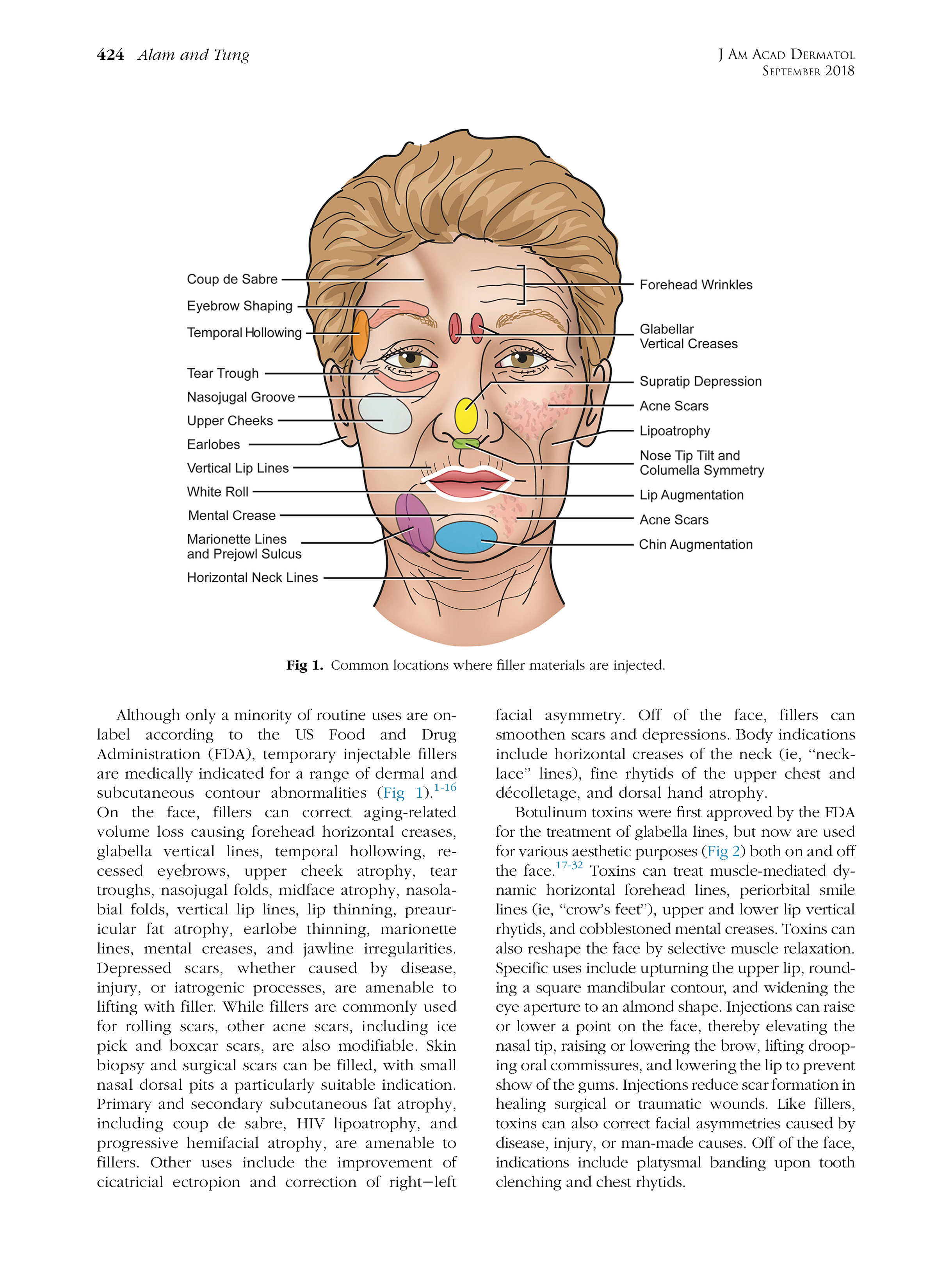
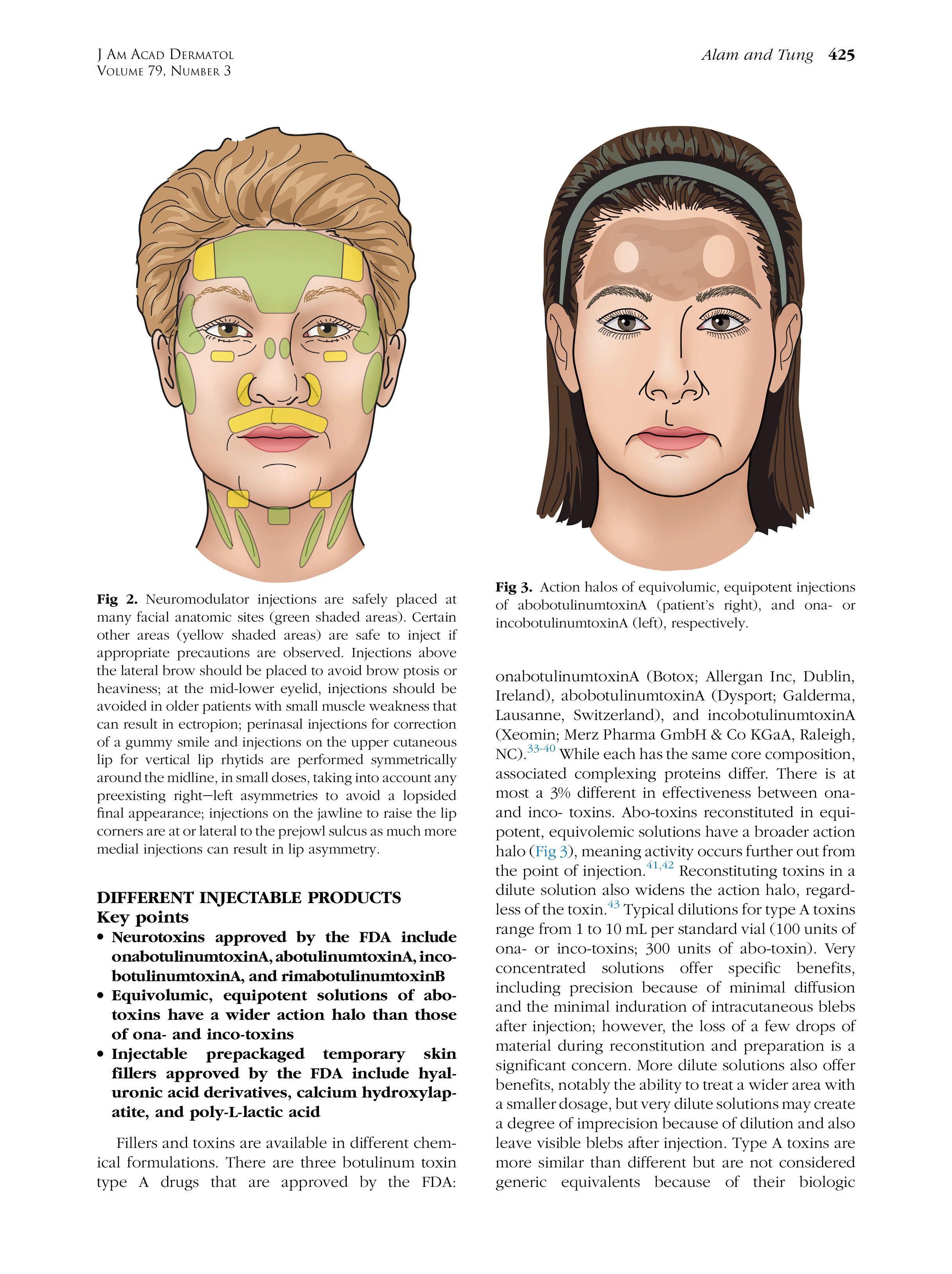
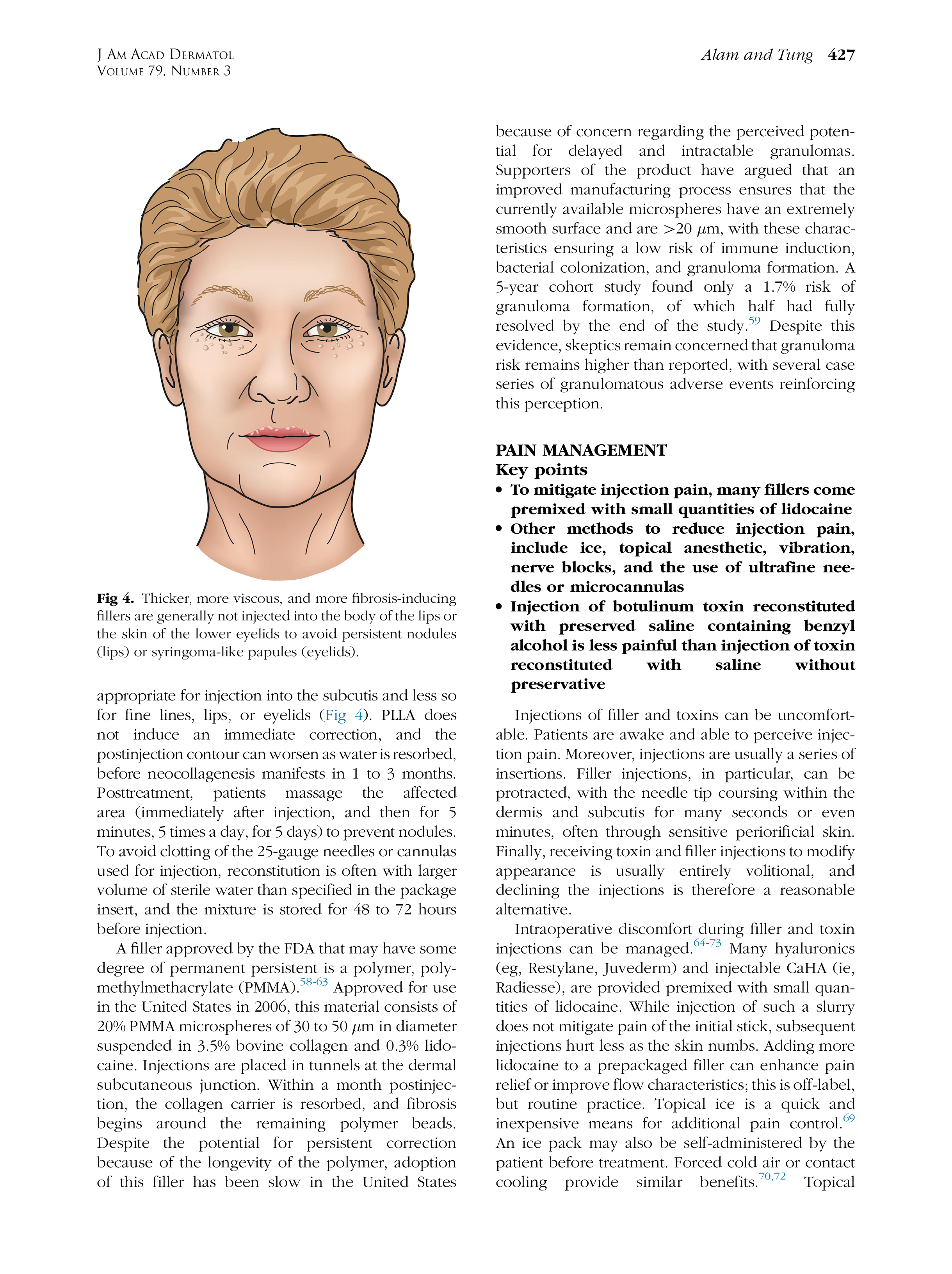
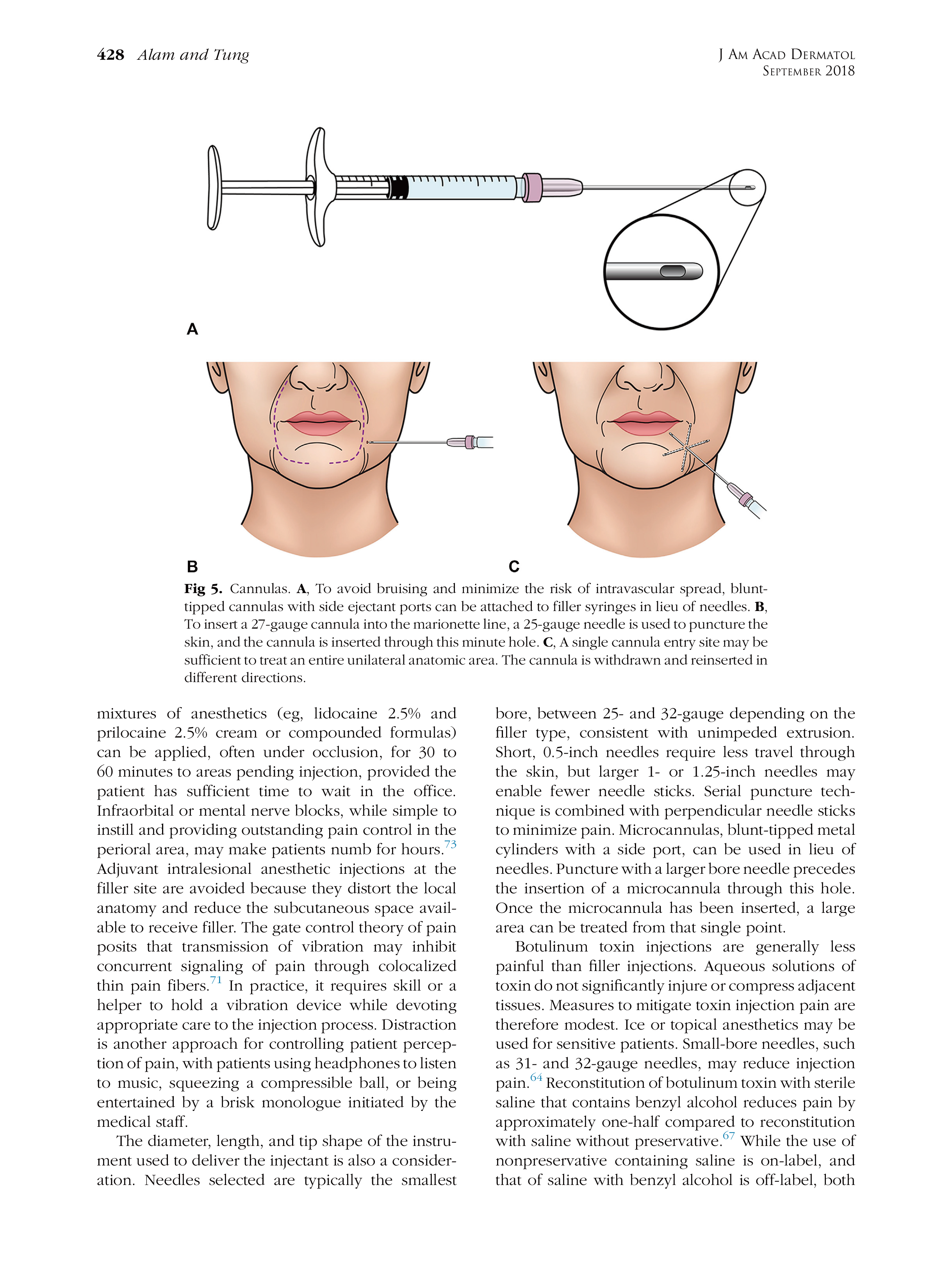




* 特别声明:
1.本栏目所收录的文献,均为已在国内外医学期刊正式发表的学术论文,其内容系独立的科学研究成果。本公司仅作为学术资料进行转载与分享,并不对文献中的观点、数据或结论进行任何形式的背书或确认。
2.本栏目内容仅供相关人士参考,不构成任何形式的医疗建议、产品推荐或治疗方案指引,所有医美项目均需前往正规机构咨询专业医生量身定制合适方案。
3.文献中涉及的医学材料或技术的功效描述,是基于特定学术观点与发现,并非对任何具体产品的功效、安全性或适应症的承诺、保证或宣传。本栏目中涉及的部分医美项目,基于产品特性,请结合所在司法辖区产品注册适应症甄别借鉴。
4.既往研究成果可能被新的科学证据所更新或替代,敬请动态甄别。
5本栏目仅供中国大陆地区年满18周岁用户浏览。

观察1例艾维岚®在修饰先天前额骨性发育不全患者中的应用效果。方法 选取2021年3月因先天前额骨性发育不全而导致真皮萎缩于我院就诊的1例患者为研究对象,选用艾维岚®进行为期2个月的额部注射治疗。观察临床疗效、不良反应发生情况、患者满意度。结果 患者前额不平整状态得到矫正,凹凸不平现象得到缓解,额部轮廓自然,线条流畅,无明显不良反应,患者对治疗效果非常满意。结论 艾维岚®额部注射用于纠正先天性前额骨性发育不全效果良好,能够有效改善面部额骨不平整现象,且具有微创、安全、有效、并发症较少的优点,值得应用。

大阴唇是女性外阴具有重要生理作用的门户结构,具有保持阴道口湿润、维持阴道自洁、防止外来污染的作用[1]。部分女性存在一定程度的先天大阴唇发育不良,外形萎缩褶皱的情况,影响生活质量。

唇部作为面部的一个重要美学单位,在面部特征中发挥着重要影响。基于唇部美学考虑,年轻的唇部状态应是莹润饱满,唇红缘线条流畅、清晰,唇体对称且无皱纹,口角微上翘[1]。而随年龄增长及外部因素的影响,唇部会逐渐呈现老化的表现,如因容量流失所致的唇红变薄、唇红缘模糊、上唇人中延长、口角下垂及口周皱纹增加等[2]。唇部年轻化治疗的目标是实现唇红容量恢复、唇纹填充、口角提升以及口周皮肤年轻化[3]。